Section 1.4 Action Plan and Implementation
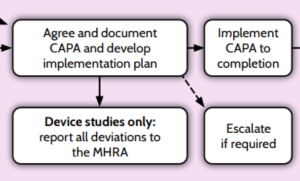
Agree and Document CAPA and Develop Implementation Plan

- The corrective and preventative actions plan should be developed by relevant members of the trial team, with support from the Quality Assurance (QA) team if relevant.
- The exact actions depend on the deviation, but could include things such as pausing trial recruitment, re-training for investigator sites, CTU staff or process changes.
- The plan should:
- Be proportionate to the deviation and actionable within a reasonable timeline.
- Clearly list all tasks that have already been implemented or are required to correct the problem and prevent recurrence.
- Clearly list who is responsible for each CAPA.
- Have appropriate deadlines for completion for each CAPA.
- The trial risk assessment should be reviewed and updated as necessary. Dependent on the review/changes, adjustments may also be needed to the other trial documents. For example, the protocol, monitoring plan or data management plan.
- The assessment process (root cause analysis) and agreed corrective and preventative actions should be clearly documented in the Trial Master File (TMF).
Escalate If Required

- Examples of escalation routes:
- Sponsor
- Chief Investigator
- Senior members of the CTU team (e.g. data management lead, statistics lead)
- Relevant investigator site(s) Research and Development Departments
- Oversight Committees (e.g. Trial Steering Committee)
- Trial funder
Implement CAPA to Completion

- Agreed CAPA should be reviewed and followed-up regularly until resolution. Part of this review may include determining if the remaining CAPA are still relevant for longer-term actions.
- Completion of the CAPA should be clearly documented in the TMF.








