In this issue
News from around the Network
- Programme Manager’s Update
- November 2025 Directors’ Meeting
- NEW Guidance and Information for CTUs
- CTU Chief Investigator Coaching Scheme
- CTU Chief Investigator Advisory Groups’ Terms of Reference
- Data Cleaning and Query Management
- Guidance on Data Reporting
- Considerations for Data Retention and Reuse
- CTUs in the News
- Training for Chief Investigators
- FACT Study Update
- Operations and T&F Group Updates
- Training & Development Opportunities
- Job Opportunities
Updates from collaborators & stakeholders

Programme Manager’s Update
As we bring this year to a close, here’s the latest edition of our network newsletter. It has been a fruitful period with a wealth of new guidance, strategic discussions with Directors, and collaborative initiatives.
A major highlight has been the release of several new national resources to support CTUs—covering data cleaning, reporting, retention and re‑use, and, importantly, the release of our new Chief Investigator Coaching Scheme guidance.
Our recent Directors’ Meeting in November was another important milestone. The focus on moving from hospital‑to‑community sparked rich discussions on trial design and the realities of decentralised delivery. Hearing from colleagues about regulatory considerations, operational challenges, and the opportunities emerging from primary care was fascinating, some of which Kerry as Network Director has already fed back to DHSC as promised.
Over the past few months, it has also been wonderful to see so many CTUs featured prominently in national media. Whether through trials addressing prostate cancer, neonatal sepsis, cancer recurrence, these features highlight the difference academic-led research makes to public health and patient outcomes, and allows us as a network to use this as evidence to highlight this to policy makers and regulators.
We are also continuing our How to Be a Great Chief Investigator programme which is going from strength to strength, with the next workshop scheduled for January 2026. We’re particularly pleased to see the enthusiasm of both new and experienced CIs who engage with this training, and the strength of peer support.
The FACTS (Flourishing As Clinical Trial Staff) Study has moved into a new phase. We are very proud to have been able to support this important work and are glad to see how much engagement there is across the Network.
Across our Operations Groups, the QA Group’s coming together to discuss readiness for new regulations, and MHRA inspections, DISOG’s national meeting and growing suite of guidance, the Monitoring Group’s tools and recommendations, and the contributions from our Statistics, Trial Management, Policy, and PPIE Groups all highlight the value of our collective expertise.
We would like to extend sincere thanks to everyone who’s contributed across all our activities —whether through meetings, consultations, working groups, or by sharing your experiences. Everything we produce together is all the stronger for it.
I hope you enjoy this issue, and we look forward to 2026!
Helen Evans
UKCRC Registered CTU Network Programme Manager

Directors’ Meeting – November 2025
On 19 November 2025, Network Directors gathered in London for a full day of strategic discussions focused on hospital-to-community research delivery and the lessons that could be learnt from existing trials work in a primary care setting.
The meeting opened with Kerry Hood (Network Director) and Helen Evans (Network Programme Manager) providing an overview of the Network’s extensive activities over the past 12 months—from engagement with policymakers to the publication of new guidance—and outlining plans for 2026.
This was followed by an update from the Network’s Clinical Director, Rustam Salman (Edinburgh CTU) on the work of the Chief Investigators’ Network Group, including the release of new resources designed to strengthen collaboration between Chief Investigators and CTUs. [Details of these resources can be found here.]
Will Cragg, Regulatory and Governance Affairs Manager at Leeds CTRU and Chair of the Network’s QA Group, highlighted key regulatory considerations and recommendations identified through a review of trials conducted outside secondary care settings. Chris Butler, Director of the Oxford Collaborative CTU, shared insights into trial delivery in primary care from both CTU and CI perspectives.



Two roundtable sessions shaped the day’s discussions:
- The first explored ways to streamline processes in the non-commercial portfolio, addressing duplication in approvals, capacity and capability bottlenecks, and inefficiencies in site set-up. Outputs from this session will be shared with the DHSC.
- The second focused on the risks and opportunities of decentralising trials and shifting delivery into community settings. Topics included regulatory and operational complexity, data integrity, digital exclusion, patient trust, and capacity in primary and social care.
The meeting concluded with a session led by Phil Evans and Morag Burton, who provided the NIHR RDN perspective on decentralising research and improving patient access.
A big thank you to our speakers who provided such excellent food for thought and everyone who shared their experiences during the roundtables and Q&A.
Work on the agenda for the May 2026 CTU Directors’ Meeting will begin soon. If you’d like to be part of the group shaping the agenda, please contact the Secretariat on regctus@leeds.ac.uk.

New Guidance for CTUs
We are exceptionally pleased to announce the release of a number of new resources for CTUs. All items are available via the links below.
We are very grateful to everyone involved in the production of these pieces.
CTU Chief Investigator Coaching Scheme
Coaching builds skills & helps put knowledge into context. This framework sets out principles for coaching first-time Chief Investigators (CIs) working with CTUs. It is designed to be adapted locally, supporting new CIs through structured guidance and mentoring.
Download your copy here.
CTU Chief Investigator Advisory Groups’ Terms of Reference
This document provides example Terms of Reference for CTU advisory groups representing the CIs perspective, offering strategic advice and strengthening collaboration between CTUs and Chief Investigators.
Download your copy here.
Data Cleaning and Query Management
Data cleaning is essential for ensuring data quality and integrity. This guidance outlines good practice for cleaning data within clinical databases and provides a useful starting point for the review of existing processes or the creation of new ones.
Download your copy here.
Data Reporting Guidance
This advisory document provides recommendations for ongoing data reporting in academic clinical trials throughout the life of a study. It covers the reporting study progress, oversight, and monitoring, including report types, potential risks, and mitigations.
Download your copy here.
Data Retention and Re-use
This document aims to provide guidance and considerations around data retention and re-use, usually, but not only, following the end of the trial (as defined in the trial protocol). It is intended to complement existing guidance on data archiving, data sharing, and anonymisation.
Download your copy here.

CTUs in the News
If we’ve missed a mention of your CTU or one of your trials, please let us know on regctus@leeds.ac.uk.
Barts CTU
Rhian Gabe was quoted in The Guardian on 17 November 2025 in relation to the TRANSFORM trial which focuses on Prostate Cancer. Read the article here. Find out more about the study here.
ICR-CTSU
The 5G study, led by The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, is the world’s first adaptive clinical trial platform for patients with brain tumours. This ground-breaking trial was featured in Episode 4 of Channel 4’s ‘Cancer Detectives. Find out more about the study here.
MRC CTU at UCL
The NeoSep1 trial featured in The Guardian on 25 November 2025. This is an international trial seeking to identify effective new antibiotic combinations for newborn babies with sepsis. Read the article here. Find out more about the study here.
Southampton CTU
Infections due to failed stents and catheters cost the NHS an estimated £2.5bn a year. On 14 November 2025, Dr Ali Mosayyebi was on BBC Radio Solent talking about the CASSETTE trial of a new urinary stent, designed to reduce infections. Find out more about the study here.
Over two thirds of children with ADHD experience sleep problems. The Sleep Buddy website has been developed by experts alongside parents and carers of children suffering ADHD-related sleep issues. It’s now being trialled in a UK-wide study. Lead researchers Prof Sam Cortese and Prof Cathy Hill spoke to BBC Radio Solent on 6 November 2026. The project was also featured on South Today. Find out more about it here.
Could a simple blood test spot the signs of cancer returning earlier than a routine scan? Episode 3 of Channel 4’s ‘Cancer Detectives featured DETECTION-2 trial, which is led by Chief Investigators Dr Becki Lee and Professor Paul Lorigan from the University of Manchester and The Christie, and run by the Southampton CTU. Find out more about the study here.
Oxford Vaccine Group (Oxford Collaborative CTU)
Professor Sir Andrew Pollard, Director of the Oxford Vaccine Group, spoke to BBC Radio 4’s Today Programme in September 2026, to discuss the £118m research funding of a new AI vaccine research programme. Listen to the clip here or find out more about the project here.

Training for Chief Investigators
The next ‘How to be a Great Chief Investigator for Clinical Trials’ workshop is scheduled to take place on 27 January 2026 and will be hosted by Barts CTU and the Pragmatic CTU. Please share these details with any current or new CIs who might be interested in further developing their practice.
The workshop is designed to equip researchers with the skills and knowledge necessary to excel as Chief Investigators, through:
~ Expert guidance from experienced Chief Investigators with a diverse range of backgrounds and specialties.
~ The opportunity to meet and gain insights from specialists at Registered CTUs, including statisticians and trial managers.
~ Interactive activities to develop practical understanding of the issues.
~ A collaborative learning environment that fosters peer support.
A key feature of the workshop is the opportunity for delegates to develop their own peer-support network, enabling ongoing collaboration and shared learning beyond the event.
Further information and a registration link can be found here.


Flourishing As Clinical Trial Staff (FACTS) Study Update
The FACTS study team have been working with clinical trial staff and other academics to develop guidance for Clinical Trials Units (CTUs) in order to help them improve employee workplace wellbeing (flourishing).
As part of the original phase of the study, we conducted surveys with staff at all 50 UKCRC-registered CTUs (n=484), focus groups (n=24) and consensus panels (n=18) to understand levels of wellbeing, develop solutions, explore implementation issues and reach an agreement on support strategies.
Using these data, we developed the FACTS guidance as a first step in addressing a significant gap in current wellbeing support for employees working in clinical research institutions.
We are now monitoring the implementation and impact of the guidance through a pre-implementation survey and a six-month follow-up survey. The pre-implementation survey has now closed to recruitment.
We would like to say a huge thank you to the 29 CTU Operational Directors who have completed the CTU-level survey, and the 432 CTU staff who completed the employee-level survey. Given the current pressures and limited resources faced by many CTUs, this level of engagement shows how much CTU colleagues value workplace wellbeing initiatives. It is more important than ever we look at how we can make often simple and small changes to the way we work to support our colleagues.
We would also like to thank (in no particular order):
~ The UKCRC Registered CTU Network for their support throughout this journey.
~ The NIHR (National Institute for Health and Care Research) Incubator for providing funding that will make our additional support package a reality for 10 CTUs across the UK.
~ Prof. Eleanor Mitchell, Dr Kirsty Sprange, Dr Louise Thomson.
~ Prof Pam Hagan BSc PhD SFHEA, Ms Lucy Carr and Dr Jodi Taylor whose support and insights have shaped FACTS at every stage.
We are pleased to note that two papers arising from the study have recently been published:
Paper 1: Flourishing and job satisfaction in employees working in UK clinical trial units: a national cross-sectional survey: https://t.co/Vdwa0XOOG3
Paper 2: Wellbeing for staff in UKCRC-registered Clinical Trials Units: Development of the Flourishing As Clinical Trial Staff (FACTS) guidance: a mixed-methods study: https://t.co/K4SQjPVMri
– Sophie Hall, Jenny Riga, Georgia Clancy (Nottingham CTU)

QA Group Update
National Meeting
The national meeting took place in Leeds on 9 October 2025.
There were two major themes for the day: CTUs’ recent MHRA inspection experiences, and preparations for the new clinical trials regulations ahead of the ‘go-live’ date in Q2 2026. The latter topic was the focus of the afternoon’s breakout session, giving CTU representatives a chance to share learning and compare preparation approaches.
There was a great atmosphere of collaboration in the room, and we are very grateful to everyone who took part in the discussions for being so open to sharing. Later in the day, we were also lucky to be joined by Michelle Gabrielle from the MHRA who provided a very useful update, including key findings from recent inspections. She also kindly answered some tough questions from delegates.
This event was slightly different to our usual meetings as we invited a number of vendors to attend. This was done both to enable delegates to meet with vendor representatives in an informal way and learn more about the products available and, importantly, so that the vendors could learn more about some of the challenges of running non-commercial clinical trials. This seemed to be well received by delegates, so we will consider running this again in 2026.
QA Clinics
During 2025, we have held three online ‘clinics’. These have been informal sessions, each with a main theme and some additional time allocated at the end for people to raise any other topics. High on the agenda has been the new clinical trials regulations. At the last session in November 2025, members shared their progress in preparing for the regulations’ implementation. There was also a discussion on the implications of the move to adopting ICH Good Clinical Practice principles in the UK regulations, and the implied link to the requirements of the first ICH GCP annex.
We will be running a short feedback survey to understand whether representatives have found these sessions helpful and would like them to continue in 2026. Respondents will also be able to suggest topics for discussion. The survey will be shared via the QA JISCMail list. If you are the QA representative for your CTU, please do share the email with colleagues locally who also attended. The more feedback the better.
Review of ICH GCP requirements
The QA Group is leading on an exercise to evaluate the requirements of the ICH GCP annexes (particularly annex 1), with a view to understanding potential challenges these might pose for academic CTUs. Once the challenges have been identified and explored, we will work collectively as a Network to establish how we can tackle these, aiming to find common solutions where possible. The QA Group and Policy Group are also engaging directly with MHRA on this topic.
GCP Inspection Preparation Guidance
In the New Year, the group will be progressing its review of its GCP Inspection Preparation Guidance. This will take into account the changes noted over the last few years, including the impact of the expanded pre-inspection dossier and the use of Microsoft Teams for sharing documents. We will also include a section on preparing for a laboratory inspection.
Running CTIMPs outside of secondary care
Work is ongoing to develop guidance on running CTIMPs in settings outside of secondary care. An outline of the main messages from the work were presented at the Directors’ meeting in November 2025. The project team are in the process of gathering case studies, working with relevant trial teams from across the Network.
Membership
We are sad to say goodbye to our departing Deputy Chair, Sara Yeats (Southampton CTU) who is retiring. It has been wonderful working with her, and we are very glad to have been able to benefit from her experience, not least in the review and refresh of the QA Oversight of Laboratories guidance which she led.
– Will Cragg (Leeds Clinical Trials Research Unit)
Data & IS Group (DISOG) Update
National meeting
The Data & IS National Meeting 2025 was an overwhelming success, attended by nearly 60 data and IS representatives from 38 UKCRC-registered CTUs.
The feedback this year was notably positive, highlighting the value of this community event. The overall event quality was rated as excellent by 100% of respondents, with attendees frequently describing the event as “superbly organised.” The organisation on the day and the administration prior to the event also received very positive feedback (with the majority rating them as excellent). Most rated the presentations as excellent (94%) and the content was generally found to be highly relevant, informative, and useful. Highlights included the sessions on MHRA Inspections (experience sharing, reflections, findings) and the (W)Agile project management talk, which were widely cited as the most useful and valuable parts of the day. Presenters were engaging and facilitated productive discussions. The networking opportunities were appreciated, and the meeting was commended as a safe space to share experiences, learn with peers, and connect with people in a similar situation.
The inclusion of vendors was supported as important for understanding the industry’s perspective and generating internal discussions, and the vendor selection was rated as very good, with interesting presentations. Following last year’s positive feedback, we increased the number of vendors attending with stands only this year to facilitate interaction during breaks.
We are pleased with the response and thank everyone who attended and contributed to the meeting’s success.
Data Management Guidance Update
The work on the academic CTU-specific data management guidance continues to be a high priority. The guidance for ‘data cleaning & querying’ and ‘data reporting’ has now been published and can be downloaded here. The guidance for ‘CRF/eCRF development’ and ‘study database lifecycle’ will be published shortly.
If you would like to be involved in future working groups, such as the planned group on ‘working with external/third-party data’ or have suggestions for future working groups: please contact the UKCRC Secretariat on regctus@leeds.ac.uk.
Upcoming Work and Suggested Topics
In 2026, we plan to continue building on the momentum of the past year with further webinars and working groups, investigating a range of new topics suggested by the community.
We have an ongoing working group regarding REDCap validation and plan to release some guidance from that group. Topics suggested for future working groups include CDISC, a greater focus on collective MHRA outcomes and solutions, and discussion around the standardisation of roles within CTUs. We also received suggestions for a MedDRA Working Group.
For future webinars, suggested topics include risk assessment and managing non-CDMS data sets. Topics put forward for future face-to-face meetings include eConsent and Randomisation, given many CTUs use bespoke solutions, as well as the practicalities of data sharing and the future of Federated Secure Data Environments/Trusted Research Environments.
We are also investigating ways to communicate more effectively with all staff working in data management and information systems in CTUs.
Please keep an eye on the mailing list for forthcoming surveys, including a survey aimed at informing the future work around MedDRA coding and opportunities to get involved.
DISOG Membership Update
One member of the Data & IS Operations Group (DISOG) has stepped down. Therefore, we are currently seeking a replacement member to specifically represent Information Systems (IS) within the group. An email with further information on this opportunity has been sent to CTU directors and IS/data management representatives.
– Amanda Loban (Sheffield CTRU), John Turgoose (Hull Health Trials Unit), & Lindsey Masters (MRC CTU at UCL)
Trial Management Group Update
National Meeting
We welcomed 93 representatives from 41 CTUs to the groups online annual meeting at the start of October. There were two main topics for the meeting which engendered some good discussion – the experiences of 4 units of recent MHRA inspections and the use of different eTMF systems. Many thanks to all those that joined the meeting to present and everyone who took part in the discussions that followed. The group are now thinking about our 2026 meeting! If you would like to share your work, please contact the Secretariat on regctus@leeds.ac.uk.
Group Activities
Resource Intensive Activities
Over the past few years the group has been investigating the activities within trials that take a lot of time or resource but are considered by Trial Managers to not add value to the quality of the trial, safety of trial participants or the trial endpoints. This included a survey of trial managers across the Network and a breakout session at one of the national meetings. The findings have now been synthesised and can be downloaded here.
Trial Roles required in Grant Applications
A paper on the trial roles required in Grant Applications is nearing being published on the UKCRC CTU resources page. It is envisaged that this will be a useful paper to share with new Chief Investigators when costing up a trial proposal to help them understand the role of different members of the trial team.
Trial Set-up
The survey that went out to CTU Operational Leads in March of this year is just on the final stages of being written up to be shared across the Network. There are some interesting findings that we hope will be useful in negotiations with funders regarding trial set-up periods.
Private Hospitals
The group are working on some guidance for including private hospitals in trials whether as full sites or undertaking only aspects of trials.
Defining new actions
The group are in the process of reviewing the Network’s Strategy for 2025-2030 to identify new deliverables that the group can lead upon – more information in the next newsletter.
Membership
Many thanks to Ana Sousa Marcelino Boshoff and Claire Cantley for their years of service and commitment to the group – include Claire having been the deputy chair of the group – as they both step down from the committee.
– Vicki Barber (Oxford Clinical Trials Research Unit) & Gina Cranswick (Edinburgh CTU)
Statistics Group Update
Webinar Series
Our webinar series continues with a wide range of topics from expert speakers. Upcoming talks will address incorporating historical information in a trial, and designing follow-up trials, with further potential webinars on the use of AI in trials and ethical challenges for clinical trial statisticians. Please keep an eye out for announcements about dates and registration. Recordings of earlier webinars can be accessed via the Secretariat.
Group Activities
The Statistics group have been involved in several activities this year, including reviewing the new UK clinical trials legislation, updating the UKCRC CTU accreditation criteria, the Future Statistician Initiative, use of AI in CTUs, a public consultation on new ICH E20 guidance for adaptive clinical trials, another on MHRA guidance on the use of external control arms based on real world data to support regulatory decisions and a review of clinical trial monitoring across CTUs. We are now developing ideas for new projects in response to the updated UKCRC CTU strategy and workplan.
Suggestions for projects the Statistics group could focus on are always welcome; please email these to the Secretariat on regctus@leeds.ac.uk.
PSI Careers Event
The Statistics group represented the UKCRC CTU network at the annual medical statistics careers fair organised by Statisticians in the Pharmaceutical Industry (PSI), which was held in Cambridge on 19 November 2025. A member of the Statistics group and two early-career statisticians from Cambridge CTUs chatted with students about statistical careers in academic CTUs. There was a great deal of interest in opportunities outside of commercial research, and for many this was the first they had heard about CTUs. We encourage all statisticians to take advantage of any opportunities, both locally and nationally, to promote the work of registered trials units.
Find out more here.
Induction and training materials for new statisticians
We had a task group compile induction checklists sent to us from 10 different CTUs into one document which any CTU can use with new trial statisticians to record on-the-job training and experience. A draft of the template was made available for comment at the national meeting in Oxford in June and a copy has been circulated to units to pilot. Please email the Secretariat on regctus@leeds.ac.uk if you would like a copy or to provide comments on piloting the checklist.
Additionally, we are collating a list of training materials which CTUs have developed or use with their new statisticians. Our aim is to create a repository which network colleagues can access and offer up new materials for. Seven CTUs responded to us last year but we are still keen to hear from a wider range of units.
Annual National Meeting
Plans are underway for the next annual national statistics meeting to be held in London, provisionally in spring 2026. Details to follow in due course. If you would be interested in sharing your work, please email regctus@leeds.ac.uk.
– Stephen Bremner (King’s CTU) & Jo Haviland (Pragmatic CTU)
Policy Group Update
The Policy Group continues to engage with key national stakeholders, including the DHSC, MHRA, and NIHR, to represent the interests of the CTU community. We also participate regularly in the HRA’s Study Set Up Partnership Board meetings to ensure ongoing input from the Network.
In the autumn, the Group coordinated the Network’s response to the MHRA consultation on the Medicines and Medical Devices Act 2021. This was part of a regular statutory review of the UK’s medicines and medical device regulatory framework undertaken to assess the operation and impact of the legislation that govern the development, authorisation, supply, and oversight of medicines and medical devices in the UK.
Representatives from the Group also attended the MHRA GCP Risk Proportionality Guidance Workshop in September 2025 to ensure that the academic community was represented in those discussions. The key take away was that the MHRA is keen to engage with the academic trials community to ensure that ICH (E6) can be implemented without adversely impacting how we conduct our trials.
– Claire Snowdon (ICR-CTSU)
Monitoring Group Update
Monitoring Clinics
25 participants joined in October for a discussion about metrics and thresholds and the changes required due to the legal changes due in April 2026. Thank you to everyone who took part.
Further sessions are planned for 2026, so ask your CTU monitoring lead for the TEAMS link if you would like to join in the conversation. You can also email s.love@ucl.ac.uk if you would like a particular subject to be covered in future clinics.
Protocol deviation route map
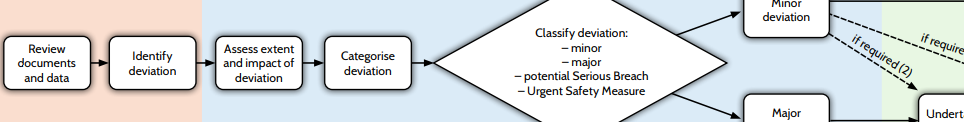
An interactive route map on protocol deviation has been added to the UKCRC clinical trial monitoring page at https://ukcrc-ctu.org.uk/clinical-trial-monitoring/. Recording and/or reporting deviations is an important method for maintaining trial oversight and ensuring that the trial is conducted as planned. Deviation collection should follow a risk proportionate approach, with specification in the protocol of important deviations. This route map details the actions in identifying, evaluating, root cause analysis, implementation of a corrective action plan and follow-up for protocol deviations in clinical trials.
Central data monitoring of clinical trials
Thank you to all of those who took part in our survey on central data monitoring. This enabled us to identify five key recommendations. We have written up and submitted the results for publication and hope to have more on this soon. We are also discussing whether we can create tools to help trials units implement the recommendations, which are:
- Consider the level of central monitoring that a trial might require at the grant application stage to ensure that sufficient resource is available
- Have a central monitoring SOP that gives an overview of the central monitoring processes at a unit and the variety of places that it is documented in e.g. the trial monitoring plan
- Train new staff (even those who may have worked at other units) in this SOP and any relevant trial specific monitoring plans as soon as possible
- Consider Protocol compliance/deviations, Patient flow (CONSORT chart), Recruitment vs predicted recruited, Eligibility issues, Consent issues, Data completeness for primary analysis, Missing CRFs, Data outliers, during risk assessment in trial set up and document who is responsible for which activities and when in a trial specific monitoring plan.
- Align central monitoring processes with other trial processes to increase efficiency
Do contact the UKCRC network at rectus@leeds.ac.uk if you want to become actively involved in any monitoring projects.
– Sharon Love (MRC CTU at UCL)
Chief Investigators Network Group (CING) Update
CING became a Network Operations Group
The second half of 2025 was a significant milestone for the CING, which consolidated its position within the Network as the seventh Operations Group. We were delighted to be given this status by the Executive Committee, which recognises the importance of the collaboration between Chief Investigators (CIs) and CTUs, and the need to nurture this relationship. The significance of this is that the CING’s focus will turn to providing opportunities for peer-to-peer support, networking, and outputs relating to the creation of training and guidance materials and sharing learning and developing best practice across CTUs. The CING will also be aligning its work with the Network’s strategic priorities in a shape that has meaning and value for the group’s remit and thematic area.
Now that the CING has made this transition, we will update our Terms of Reference to be consistent with the aims of Operations Groups to:
- Identify challenges and develop solutions together
- Highlight challenges which require a policy response
- Identify opportunities to improve efficient trial conduct
- Share best practice and developing practical guidance on good research practice and quality standards for non-commercial trials
- Contribute to national and internationally led new initiatives within the clinical research community; and
- Represent the views of Registered CTUs
CING increased its diversity
We were pleased with the outcome of the open call to CTUs to nominate additional members of the CING, following the departures of Vikki Wylde and Alistair Hay, who we thank for their contributions. The call for applications signalled our desire to increase the diversity of the group, so we were particularly pleased that the Executive Committee approved the appointment of CIs representing Northern Ireland, NMAHP colleagues, social care, primary care, and an interest in postgraduate education. At CING’s last meeting on 4 November 2025, we welcomed these new members to the group, whose full membership is listed here: https://ukcrc-ctu.org.uk/chief-investigator-network-group/.
New CTU Chief Investigator Coaching Scheme launched
In October 2025, we published a framework and the key principles for coaching for ‘new’ (i.e. first-time) CIs of clinical trials conducted with CTUs. The intention is for this document to be adapted by registered CTUs in the UKCRC Network according to their local composition, capacity, institutional support available, and extent of experienced CI involvement in the CTU. The Word document, which can be adapted by CTUs to suit their own needs, is available here: https://ukcrc-ctu.org.uk/chief-investigators/
Registration is open for the next How To Be A Great CI (HTBAGCI) workshop!
The Network has continued to support the HTBAGCI workshops to occur three times per year, with CPD/CME accreditation. The last workshop was a pan-Scotland initiative, hosted by the Glasgow CTU on 9th November. As usual, there were ~30 delegates and faculty, with much-valued accounts of the lived experience of a variety of CIs and opportunities for face-to-face networking.
The next workshop will be at Queen Mary University of London on 27th January 2026. Speakers include: Prof. Beth Stuart (QMUL), Prof. Rhian Gabe (QMUL), Ms. Laura White (QMUL), Tahera Hussain (PhD), Dr. Alison May Berner (QMUL), Ms. Rebecca Newton (QMUL), Prof. Steph Taylor (QMUL), Prof. Rupert Pearse (NIHR), Prof. Paul Little (University of Southampton). The full programme and registration are available here: https://ukcrc-ctu.org.uk/how-to-be-a-great-chief-investigator-for-clinical-trials-one-day-workshop/.
Future plans
At the last CTU Directors’ meeting in London on 19 November 2025, I outlined the future plans for CING, which include:
- Updating the Network’s checklist for CI engagement with a CTU
- Beginning work on an “Associate CI” scheme
- Scoping an educational resource for CIs
- Supporting How To Be A Great CI courses (3x/year) – https://ukcrc-ctu.org.uk/how-to-be-a-great-chief-investigator-for-clinical-trials-one-day-workshop/
– Rustam Al-Shahi Salman (Edinburgh CTU)
PPI&E Group Update
The PPIE Task and Finish Group would like to thank all the UKCRC Registered Trials Unit staff who responded to the Training Needs Survey. This survey collected information from all on the level of training currently offered and identified the training needs of trial management teams in relation to PPI&E and provide recommendations for future training resources and materials. The responses to the survey have now been analysed by the T&F group, and the results will be shared with Centre Directors and other relevant CTU staff in the near future.
After much consideration, the T&F group have decided that despite efforts to collect materials, a central repository of documents will not be possible. This is due mainly to the fact that keeping this up to date is a difficult task as documents are updated constantly. The group will be sharing a list of recommended public domain resources in the survey report.
– Sally Hopewell (Oxford Clinical Trials Research Unit) and Laura Farrelly (MRC CTU at UCL)

Training & Development Opportunities
The Network, and its member CTUs, are committed to sharing their knowledge of best practice in clinical trials research from the absolute fundamentals of trial development and management to the use of the latest novel trial designs.
You can find out about some of the upcoming learning and development opportunities taking place across the Network here.

Job Opportunities Across the Network
Working in an an academic or non-commercial CTU can offer you exciting opportunities that may not be available to you elsewhere. These range from publishing your research, presenting at national or international conferences, or teaching the next generation of researchers, as well as making a positive difference to patient and public health.
You can find out more about current vacancies across the Network here.
THE INFORMATION INCLUDED BELOW HAS BEEN PROVIDED BY PARTNER ORGANISATIONS
AND IS INCLUDED WITHOUT EDITS

UKTMN Update
UKTMN Annual Conference 2026
The UKTMN’s next annual conference is scheduled to take place in Cardiff on 9 June 2026. A draft programme has recently been announced to UKTMN members and registration will open on 5 January 2026. The programme includes keynote presentations from Alice Mortlock (DHSC) and Paula Williamson (TMRP), parallel sessions, trial management presentations, posters, and networking. Find out more here.

UKTMN ASPIRE leadership programme
The ASPIRE leadership programme has been specifically tailored for trial management professionals. Cohort 2 is ongoing and due to complete in February 2026. Applications for Cohort 3 will open in Spring 2026, with an anticipated start date of September 2026. Learn more about the programme here.

UKTMN Award Scheme
Nominations for the UKTMN Awards opened in October 2025, and we were pleased receive a record number of nominations. This year saw the introduction of 6 new award categories, expanding on the previous Trial Manager of the Year award – providing more opportunities to recognise and celebrate trial management professionals. We received 73 nominations across 7 categories. The winners will be announced during week commencing 15 December 2025. All winners will be awarded a free place at the UKTMN Annual Conference 2026. Find out more about the award scheme here.
Commentary paper published in Trials Journal
A commentary paper authored by the UKTMN Professional Development group entitled Supporting the professional development of trial managers: how can you help? was published in Trials in October 2026. This paper provides information to those who manage trial management professionals, focused on ways in which professional development can be supported.
Recent UKTMN training course
On 6 & 7 November 2025, the UKTMN held an ‘Introduction to statistics for trial management professionals’ course in collaboration with the Nottingham Clinical Trials Unit. Work is underway to schedule UKTMN training for 2026, including the introduction of some new topic areas.
Webinars
There have been three UKTMN member webinars held since September 2025 – ‘How could we layer information to make it easier for trial participants to digest?’, ‘Using animation to boost engagement with research.’ and ‘Managing legacy samples’. Recordings of all webinars are available via the UKTMN website.
New UKTMN Work Plan
Work is underway to finalise the UKTMN Work Plan to align with the UKTMN Strategy 2025-2029, released in Autumn 2025. The strategy outlines the mission, vision and key strategic aims of the UKTMN.

NIHR Update
NIHR leadership webinars and e-learning modules
NIHR provides a range of leadership development resources aimed at enhancing the leadership and management skills of health and care research professionals. They support you whether you are a new or an experienced leader. You can access them by our webinars or e-learning modules. Topics range from hybrid working, strategic awareness, public speaking and much more. Find out more.
Cultivate and build your networks
A new e-learning module has been launched by the National Institute for Health and Care Research which will help you evaluate and refine your networks using a four-step strategic framework.
It will explain how to identify opportunities to diversify and grow your network and share a practical networking toolkit to build a wider network across your own organisation and beyond. Find out more on NIHR Learn
NIHR RDN strategic plan sets ambitions for the next 5 years
The NIHR Research Delivery Network (RDN) has developed a strategic plan for 2025 to 2030, after extensive collaboration across the research community.
The plan sets out how the NIHR RDN will deliver on its primary purposes and focus its activities in supporting the government’s health and growth missions by delivering on the ‘3 shifts’ outlined in Fit for the future: 10 Year Health Plan for England. It will also support the delivery of the Life Sciences Sector Plan vision to be at the forefront of global innovation. The plan outlines how the NIHR RDN will work as a partner in the wider health and care system to deliver against commitments in the NIHR’s 7 strategic priority areas:
- building on lessons from the COVID-19 response.
- strengthening preventative, public health, and social care research.
- improving care for people with multiple long-term conditions
- expanding clinical research to under-served regions and communities.
- embedding equality, diversity, and inclusion.
- strengthening careers for research delivery staff.
- expanding collaboration with the life sciences industry.
To learn more, watch this video about the Strategic Plan which includes messages from RDN senior leaders, workforce, and a dedicated public partner.
NIHR RDN announces latest PRES results with more children and young people making their voices heard
2024/25 marks the tenth anniversary of the Participant in Research Experience Survey (PRES)
and this year children and young people under the age of 16, are increasingly making their voices heard when it comes to research, the results show.
There were 2,604 responses this year, the highest ever number of responses from this cohort, and a 40% increase on 2023/24.
Of the children and young people who took part, 92% felt that their participation was valued by research staff.
The overwhelming majority of people of all ages who took part in health and care research reported a positive experience. 91% of adults and 87% of children and young people said they would be happy to take part in research again.
A total of 32,268 responses were received across both surveys, with participation remaining stable despite the transition from 15 Local Clinical Research Networks (LCRNs) to 12 Regional Research Delivery Networks (RRDNs).
Headline findings:
- 91% of adults and 87% of children and young people said they would be happy to take part in research again
- 96% of adults and 95% of children and young people felt they were treated with kindness, courtesy, and respect
- 94% of adults and 92% of children and young people felt well-informed at the start of a study
- Over 93% of adults and 92% of children and young people felt valued for their participation
Read the full results on the NIHR website.
What this means for us
- retention and trust remain strong – a high willingness to take part again indicates confidence in NIHR-supported research
- first-time participants are a significant group – nearly 70% of adults were new to research, underlining the importance of clear communication and ongoing support
- young people’s involvement is growing – a record number of children and young people’s responses help us better understand their needs, but further work is needed to ensure ongoing engagement
- Operational focus – improvements are needed in how we:
- communicate study results back to participants.
- provide regular study updates.
- ensure clarity on points of contact for participants.
Examples of PRES impact
Below are some examples of how feedback received from PRES benefits research delivery.
LOLIPOP study (reaching South Asian communities)
Participant feedback led to changes such as snacks at clinics, clearer privacy signage, and pre-appointment calls/text reminders. These adjustments improved the participant experience at low cost.
Accessibility trial (Recite Me toolbar)
In 2024/25, the RDN trialled the use of the Recite Me accessibility toolbar as part of an online option to complete PRES. Over 6,800 PRES respondents used these tools, such as screen readers, translation, and style changes, providing them with inclusive options to engage with the survey. The Recite Me tool will now be a standard option for digital PRES, improving inclusivity.
Implicit Bias in Research Delivery training
PRES feedback highlighted that some participants felt uncomfortable with certain questions and language. In response, the Research Delivery Network developed a new training course, focusing on gender, sexuality, disability, and neurodiversity, to help staff use more inclusive communication and ensure that participants feel welcome and valued.
For a full copy of the PRES report or queries, contact the Public Partnership team: rdncc.ppt@leeds.ac.uk
UK Commercial Costing Reference Group Autumn Meeting Summary
The group provides cross-sector oversight of the UK interactive Costing Tool content and meets twice a year. The autumn session covered:
Areas for Refinement
– Pharmacy and primary care activities are under review to support revised resourcing for 26/27 update, with unused activities being cleared out and new tariff activities for NHS blood and transplant being collated.
Quality drives consistency
– The persistent issue of site cost coverage escalations were discussed. It was agreed that efforts should concentrate on enhancing the quality of initial costings and optimising existing system-wide resources and skills to mitigate these escalations.
Workforce empowered by digital enhancements
– Proposed digital development of the existing tool, supported by the RD forum’s exploration of robust workforce learning, aims to enhance submission quality through the auto-population of activities aligned with current written guidance. This technological advancement is expected to provide greater assurance in activity capture, thereby accelerating setup times.
WATCH: Driving research delivery with data: How to supercharge your feasibility, site identification and site selection https://www.brighttalk.com/webcast/6833/650823
The NIHR hosted webinar describes the UK wide site identification service for commercial research. This service facilitates visibility of all new commercial study opportunities and a standardised mechanism quickly express interest.
The impact of this new, all-encompassing approach is speeding up and spreading out research opportunities by highlighting the research capacity and capability of the whole health and care system.
Contact your Regional Research Delivery Network or Devolved Administration office to find out more.
SIGN UP – Shape the digital services and systems supporting research
The NIHR is on a mission to build the best user experiences through innovative and inclusive digital services.
We are working on a programme to improve the process of reporting health and social care research management data across the UK. This will involve connecting systems to allow better sharing and visibility of data across organisations involved in the funding, approval and infrastructure support of research in the UK.
This will help us track studies throughout their lifecycle and reduce any unnecessary administration burden placed on researchers and organisations.
Sign- up here to get involved and shape the future of the systems and services you use: https://www.nihr.ac.uk/help-us-build-more-connected-research-ecosystem
Applications for the Strategic and Wider Care Settings funding calls 2026/27
Would you like to help those you care for take part in research that could benefit them?
NHS and wider care setting organisations are invited to apply for 2 new funding calls for 2026/27, offered by the NIHR Research Delivery Network (RDN). The funding calls are aimed at helping to improve access to research opportunities for health and care staff, and those they care for.
The 2 funding calls are for:
- Strategic Funding
- Wider Care Settings (WCS) Funding
Both funding calls also aim to support the delivery of the 3 government shifts identified in their 10 Year Health Plan (hospital to community, analogue to digital, sickness to prevention).
Applications for both funding calls will open in stages across the Regional RDNs (RRDNs) over the course of 2 weeks, starting Tuesday 18 November 2025.







