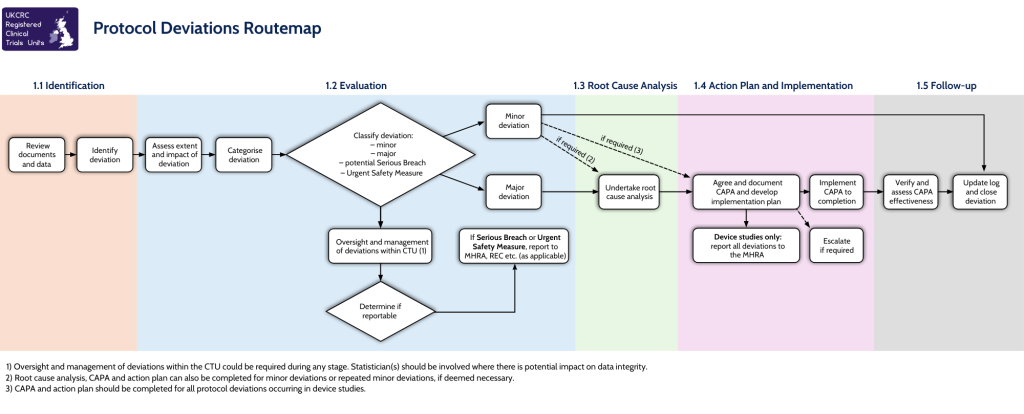
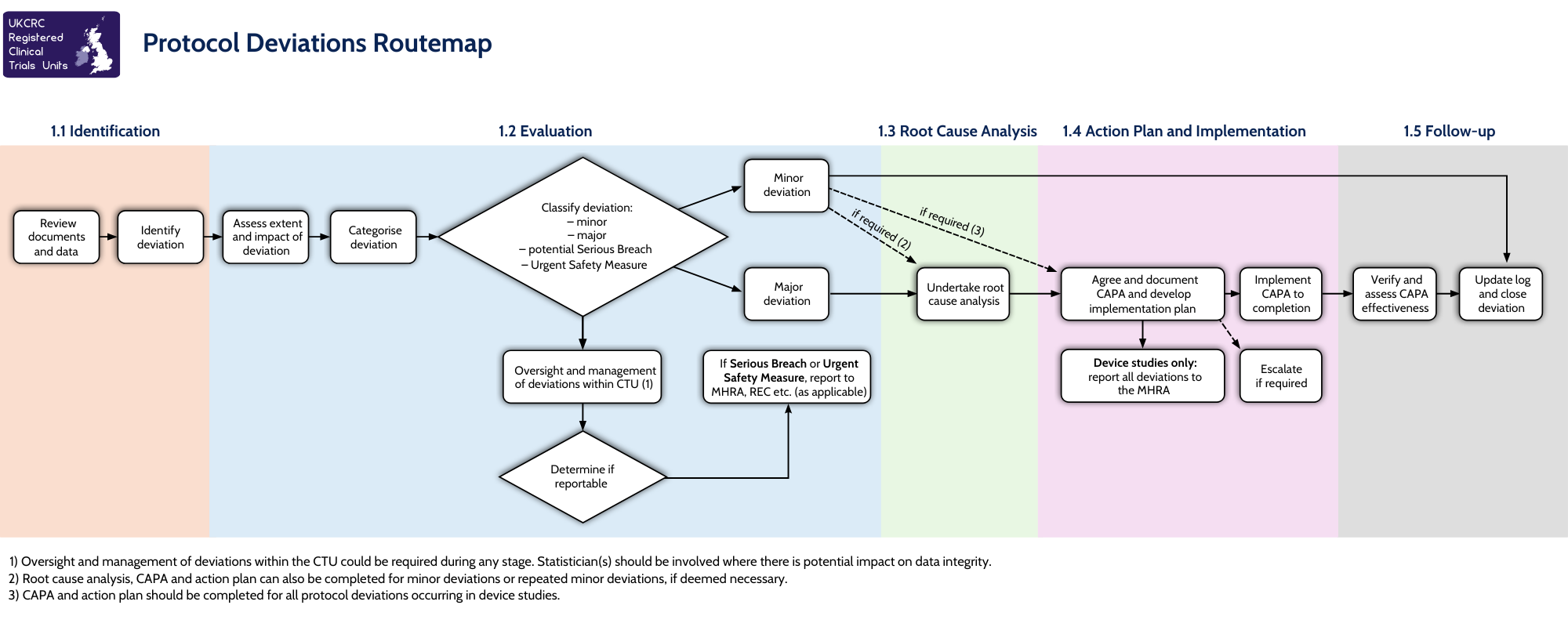
As we approach World Environment Day on 5th June, we are proud to mark one year since becoming a Supporter of the Concordat for the Environmental Sustainability of Research & Innovation Practice. This milestone highlights our ongoing dedication to embedding environmental sustainability throughout the clinical trials landscape.
In signing the Concordat, we committed to action across three key priority areas: leadership and system change, emissions from academic travel, and collaborations and partnerships.
Leadership and system change
We recognise that long-term research quality and impact depends on sustainable practices.
Over the past 12 months, we have been developing our Strategy for 2025–2030, ensuring that sustainability is not only reflected but embedded within our long-term goals. Our commitment to responsible, environmentally conscious research remains central to our strategic direction and future ambitions.
The Network also supports strengthening business models for long-term resilience while embedding environmental sustainability in all activities.
Read more about our strategy here.
Reducing emissions from academic travel
As a Network, we have reduced the number of face-to-face national meetings and transitioned to providing digital materials for all our events. Where possible we combine meetings to remove the need for multiple journeys.
We have expanded our programme of webinars, roundtables, and discipline-specific online clinics, enabling more CTU staff to access shared learning and peer support without the need for travel. This shift has not only lowered our environmental impact but also greatly improved accessibility and inclusivity across the Network.
In the last 12 months, our online programme has included 5 clinics, 6 webinars, 12 roundtables, and 15 user group meetings – allowing around 1200 people to share their knowledge and experiences without additional travel.
Collaborations and partnerships
Through collaboration and knowledge-sharing, we are supporting CTUs in advancing sustainable research practices — both by improving current systems and processes, and by exploring innovative approaches for the future.
Joint initiatives focused on simplifying and decentralising trials, harnessing emerging tools like AI, and promoting remote alternatives for activities such as trial monitoring, enable our members to benefit from shared expertise. This collective approach helps reduce duplication of effort and resources, driving more efficient and environmentally conscious research.
We are also proud to play our part in the work of wider groups in developing policies and practices that support our sustainability goals. This includes supporting the development and piloting of a carbon footprinting tool for clinical trials, led by two of our member CTUs in as part of the TMRP’s Greener Trials Group.
The future
We look forward to continuing our collaborative efforts to advance more sustainable clinical research over the next 12 months and beyond.
Together we can make a difference!
Find out more
~ To learn more about the aims and ambitions of the Concordat click here.
~ To download the Network’s official letter of support from 2024 please click here.
~ You can find out more about World Environment Day, and this year’s call for collective action to tackle plastic pollution, here.